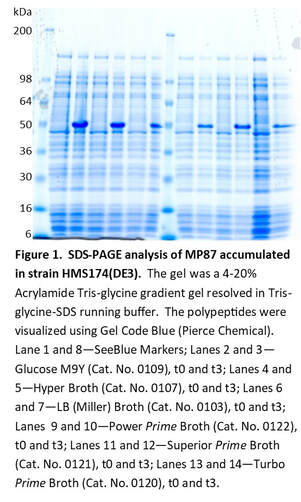
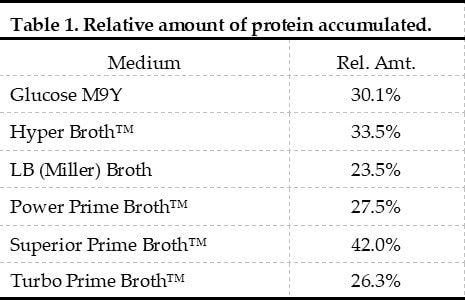
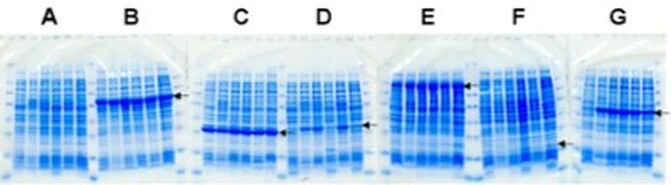
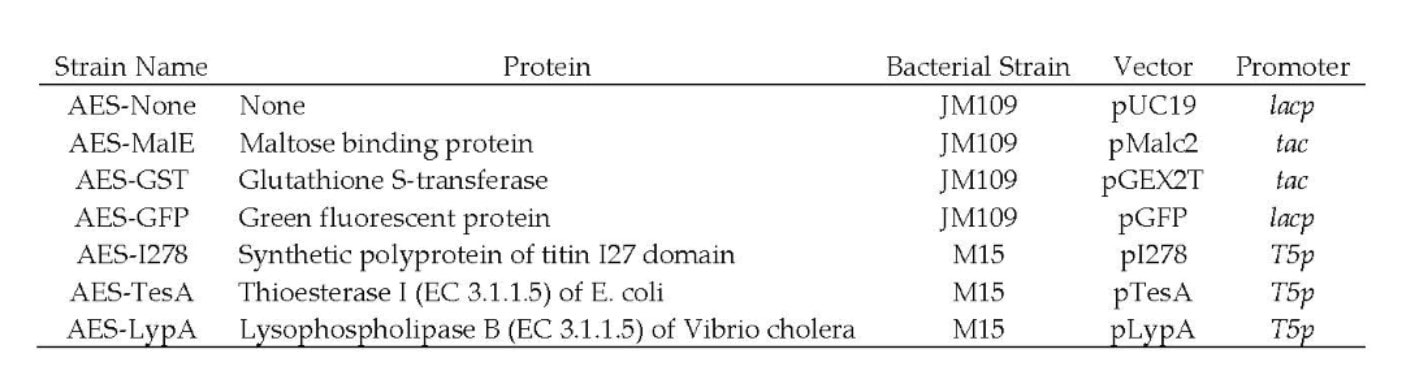
Examples of Expression Media Screens
Protein Expression | Cell Culture Products | Specialty Proteins | Enzyme Assays | Manufacturing Services
Expression Media: Kits LB and APF LB Broth™ Turbo and Turbo Prime Broth™ Superior and Superior Prime Broth™
Power and Power Prime Broth™ Hyper Broth™ Glucose M9Y
Accessory Expression Products: Atholate™ Augmedium™ and LB Booster™
Power and Power Prime Broth™ Hyper Broth™ Glucose M9Y
Accessory Expression Products: Atholate™ Augmedium™ and LB Booster™